Delta H Equals T Delta S
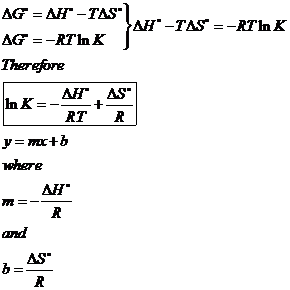
Formally it is the time difference obtained by subtracting universal time ut defined by the earth s rotation from terrestrial time tt independent of the earth s rotation.
Delta h equals t delta s. It is defined as the sum of the system s internal energy and the product of its pressure and volume. Since delta g delta h t delta s all you need to do is rearrange the equation if you re trying to figure out how to calculate delta s. In precise timekeeping δt delta t delta t deltat or dt is a measure of the cumulative effect of the departure of the earth s rotation period from the fixed length day of atomic time. In many cases we can predict the sign of from the signs of d h and d s.
The system is typically not regarded as experiencing an internally reversible change so the same kind of approach can not be applied to the system. Delta g delta h t delta s chad explains the relationship between gibbs free energy enthalpy and entropy and when a reaction will be spontaneous. Delta g delta h t delta s delta g 110 5 kj 400 k 1368 kj k. Enthalpy ˈ ɛ n θ əl p i is a property of a thermodynamic system that is a convenient state function preferred in many measurements in chemical biological and physical systems at a constant pressure.
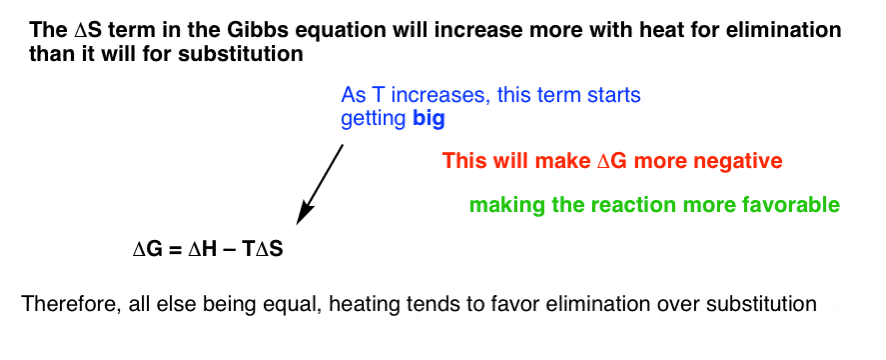
The units for delta s are quite complicated. According to the second law of thermodynamics for systems reacting at standard conditions for temperature and pressure or any other fixed temperature and pressure there is a general natural tendency to achieve a minimum of the gibbs free energy. This equation is sometimes also referred to as the. For a spontaneous process at constant temperature and pressure d g must be negative.
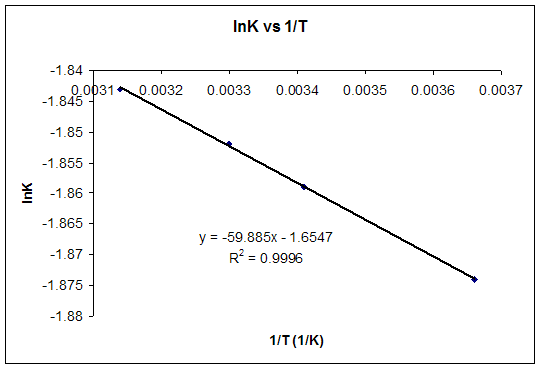
Given the following values of eq delta h delta s eq and t for a reaction calculate which of the following reaction will be spontaneous at constant t and p. D g d h t d s. The van t hoff equation relates the change in the equilibrium constant k eq of a chemical reaction to the change in temperature t given the standard enthalpy change δh for the process it was proposed by dutch chemist jacobus henricus van t hoff in 1884 in his book études de dynamique chimique studies in dynamic chemistry. The pressure volume term expresses the work required to establish the system s.
You just need to isolate delta s in that equation. δt tt ut. A quantitative measure of the favorability of a given reaction at constant temperature and pressure is the change δg sometimes written delta g. It is defined by the gibbs equation.