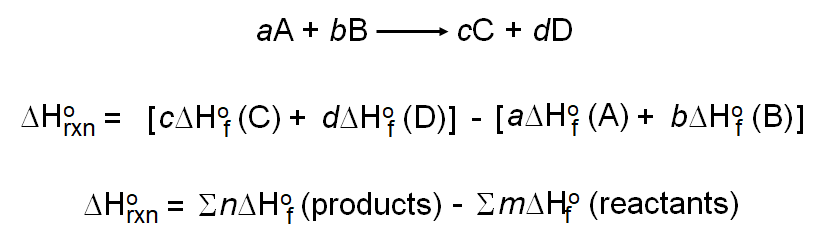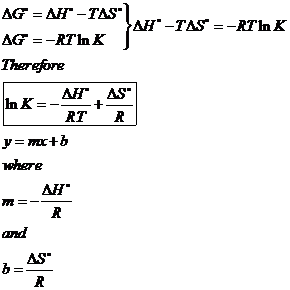Delta H Reaction Equation

H rxn kj.
Delta h reaction equation. 2 hcn g agcn s ag s h2 g 3 cn s δ h 113 kj mol. Because 2 mol of al are consumed in the balanced chemical equation we divide 851 5 kj by 2. As an example let s say we want to find the enthalpy of reaction for the formation of water from hydrogen and oxygen. The van t hoff equation relates the change in the equilibrium constant k eq of a chemical reaction to the change in temperature t given the standard enthalpy change δh for the process it was proposed by dutch chemist jacobus henricus van t hoff in 1884 in his book études de dynamique chimique studies in dynamic chemistry.
Bond enthalpy and enthalpy of reaction. δ hf of cn s 101 kj mol do not forget to divide by the 3 coefficient posted in chemistry. When a value for δh in kilojoules rather than kilojoules per mole is written after the reaction as in equation ref 5 4 10 it is the value of δh corresponding to the reaction of the. The enthalpy of reaction is calculated under standard conditions stp.
To find h for a reaction first identify its products and reactants. This equation is sometimes also referred to as the. Hess s law and reaction enthalpy change. 2015 ap chemistry free response 7.
Use the δ h and balanced chemical equation below and calculate the δ hf of cn s. Another more detailed form of the standard enthalpy of reaction includes the use of the standard enthalpy of formation δh º f. In this equation h 2 and o 2 are the reactants and h 2 o is the product. δg δh tδs.
The relationship holds true under standard conditions or under non standard conditions. However for most chemical reactions the work term p δv is much smaller than the internal energy change δu which is approximately equal to δh. Bond enthalpy and enthalpy of reaction. δgo δho tδso.
Bond enthalpy and enthalpy of reaction. The change in gibbs free energy δg for a system depends upon the change in enthalpy δh and the change in entropy δs according to the following equation. δh ominus sum delta v p delta h ominus f products sum delta v r delta h ominus f. As an example for the combustion of carbon monoxide 2 co g o 2 g 2 co 2 g δ h 566 0 kj and δ u 563 5 kj.
Latex delta h ominus rxn sum delta h ominus f text products sum delta h ominus f text reactants latex. Thus δh 851 5 kj mol of fe 2 o 3 we can also describe δh for the reaction as 425 8 kj mol of al. 6co2 g 6h2o l c6h12o6 s 6o2 g it s now asking me to calculate the delta h rxn. 2h 2 hydrogen o 2 oxygen 2h 2 o water.
The relationship between δg δh and δs.