Graphite And Diamond Atomic Structure

Diamond and also graphite are chemically the same both made up of theelement carbon however they have entirely different atomic and also crystalframeworks.
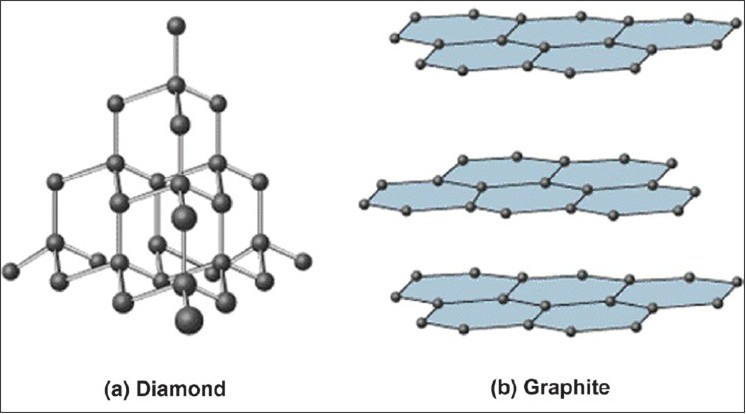
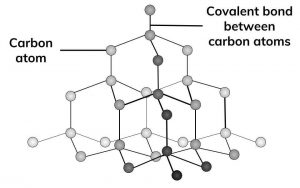
Graphite and diamond atomic structure. Introduction to diamond and graphite. Makes diamond useful for cutting tools such as diamond tipped glass cutters and oil rig drills. You can think of graphite rather. In diamond all the carbon atoms have strong chemical bonds to four other carbon atoms making perfect tetrahedra on and on throughout the crystal.
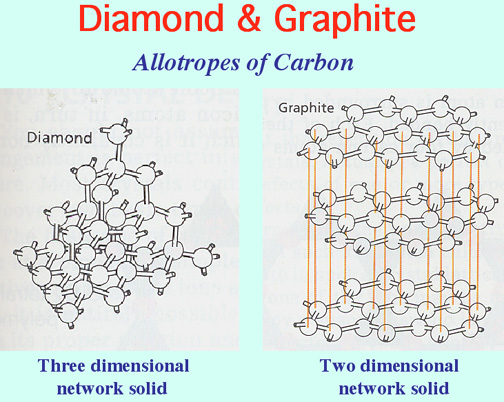
Graphite has a high melting point similar to that of diamond. Close packing in all directions of atoms in the diamond explain its great hardness. Graphite has a giant covalent structure in which. Diamond and graphite both are known as the allotropes of carbon.
In these allotropes of carbon the atoms consisting of carbon atoms in that of the diamond and graphite are bound together by strong covalent bonds with different. These minerals in general are known to be as polymorphs having the same type of chemistry but of the various crystalline structures. In order to melt graphite it isn t enough to loosen one sheet from another. It has a soft slippery feel and is used in pencils and as a dry lubricant for things like locks.
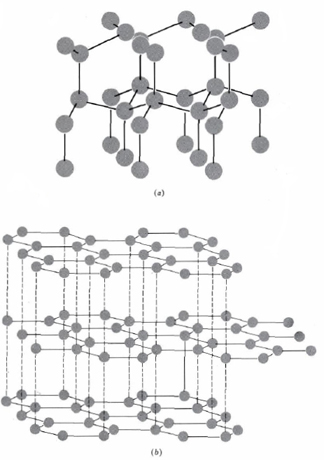
In graphite atoms within a plane are closely packed but much weaker bonding between the planes results in a soft mineral with good cleavage. Diamond left and graphite right are both made of carbon atoms but arranged in different ways. Diamond atoms have a rigid 3 dimensional structure with each atomcarefully loaded with each other as well as connected to 4 other carbon atoms.